
Info for UM Clinicians
Most comprehensive prognostic test:
Reports: We strive to provide as much useful information as possible. World-renowned medical experts contribute to cases involving complex interpretation.
Re-tests: If no mutation is found, we bank any remaining DNA. In the future, when new science or test methods are available, we will contact referring specialists to offer to re-test. For any new findings, we re-issue our report at no added charge.
Clinically appropriate turn-around time: Turn-around time is 3 to 6 weeks.
Certified lab: Our lab is fully accredited by the Ontario Lab Accreditation (OLA) Program and College of American Pathologists (CAP), registered under CLIA ‘88 legislation and compliant with ISO 15189:2012. We are a member of the European Molecular Quality Network (EMQN).
Service excellence: Impact is committed to exceptional customer service. Our team provides test order support so you can spend more time with your patients.
Logistics: Impact provides genetic testing services to about 25 countries and samples are routinely shipped from across the world without disruption. We provide the necessary paperwork and recommend a courier service that will reliably deliver the samples.
Impact Genetics’ Uveal Melanoma Test Description
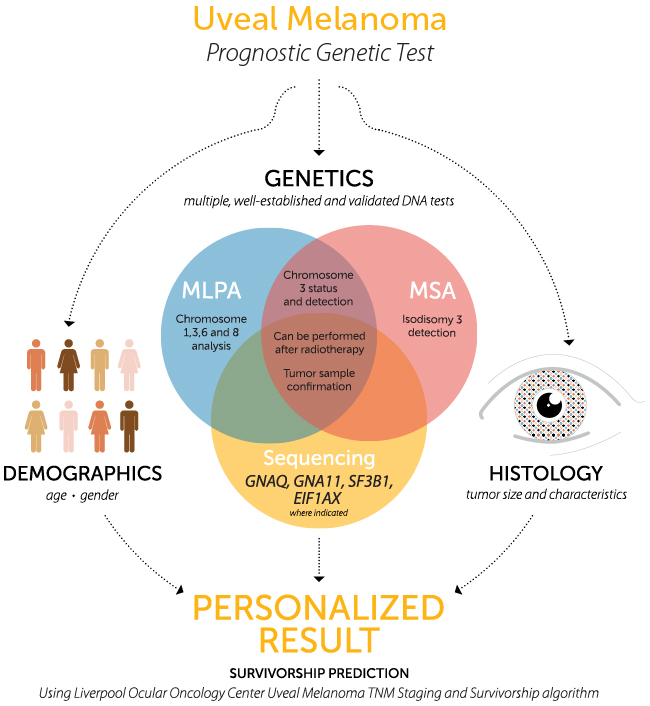
- Copy number testing using MLPA on chromosomes 1p, 3, 6 and 8 to detect monosomy, disomy and trisomy.
- Microsatellite analysis (MSA) on chromosome 3 to detect loss of a chromosome copy and isodisomy.
- Sequencing GNAQ and GNA11 to detect frequently occurring mutations in UM tumor for confirmation of tumor sampling.
Sample requirements
- 200 ng of DNA is preferred. Testing may be possible on less than 100 ng of DNA.
- Tumor samples for DNA analysis can be fresh or frozen. Samples can be safely sent with up to 2 days in transit.
- A biopsy may be taken at the time of plaque insertion or on the last day of proton beam radiotherapy.
- A biopsy may be taken from an enucleated eye.

