
News & Announcements
March 3rd, 2020
Impact Genetics is Moving

The Impact Genetics molecular laboratory is moving to a new location.
The address of our new location is:
115 Midair Court
Brampton, Ontario, Canada
L6T 5M3
All shipments should now go to this new address.
Please proceed to destroy old requisitions immediately and replace with updated
requisitions which can be found in the Order a Test section of our website.
Our phone numbers, email addresses, and website location have not changed.
Please feel free to contact us directly with any questions.
Toll Free: 877.624.9769
Local: 647.478.4902
Email: info@impactgenetics.com
March 13th, 2018
Canadian-Developed Genetic Test Sheds Light on Epilepsy

An individual’s genetics play a part in over 50 per cent of epilepsy cases. With an estimated 200,000 Canadians
living with epilepsy, many are unaware if there is a link between their genetics and their disorder. A new cutting-edge
test created by a team of Canadian researchers at London Health Sciences Centre (LHSC), and offered across
North America by Dynacare, is helping epilepsy patients uncover if they have an underlying genetic cause of
disease, and to use this information to better inform treatment.
The Epilepsy Gene Panel test is offered by the Dynacare Impact Genetics lab out of Bowmanville, Ontario with
interpretation provided by the LHSC team. The test comes in two forms: the Comprehensive Panel, which looks at
69 genes associated with epilepsy; and the Management Impact Panel which targets 16 genes associated with
treatable metabolic disorders in which seizures can occur.
The Epilepsy Gene Panel tests provide a highly accurate assessment of changes in genes associated with
epileptic disorders. It is important for those living with epilepsy to discover if they have an underlying genetic
condition causing their epilepsy so they can:
• explain comorbidities (the simultaneous presence of two diseases or conditions in a patient);
• inform prognosis;
• define risks to relatives and future children;
• manage their epilepsy better, and;
• influence treatment choices.
The test was developed by Dr. Bekim Sadikovic and the molecular genetics team at the London Health Sciences
Centre (LHSC). All patient samples (except Ontario which continues to be tested at LHSC) are sent to Dynacare
for testing and informatics and reporting are provided by LHSC. The test is provided under CAP/CLIA and ISOO
quality standards and sold by Dynacare, a LabCorp company.
Dynacare is continually expanding its internationally-recognized menu of genetic test solutions, and is
searching for additional Canadian innovations and partnerships to bring new and unique genetic test solutions
to the Canadian, US and international markets.
Contact Information:
Franny Jewett
Dynacare
jewettf@dynacare.ca
1.877.624.9769 x 5510
February 26th, 2018
Impact Genetics is excited to announce that our Epilepsy Gene Panel testing is now available!

About half of all patients with epilepsy have an identifiable genetic component. An accurate genetic diagnosis
can optimize patient care by providing valuable information regarding prognosis, comorbidities,
treatment options and risks to relatives.
Our new Comprehensive Epilepsy Gene Panel addresses the majority of known genetic causes of
epilepsy in a single test and is appropriate for patients with epileptic seizures of unknown etiology.
The 69 genes in this panel are associated with disorders for which seizure activity is a key clinical feature
and includes 16 genes that have direct therapeutic implications.
Epilepsy Gene Panel testing at Impact Genetics includes:
• Advanced next-generation sequencing (NGS) technology that detects sequence and copy number
changes with 99.9% analytic sensitivity
• Confirmation of all reportable variants by an alternate testing method (e.g. Sanger sequencing or MLPA)
• Free parental testing for interpretation of variants of uncertain significance (VUS)
Educational webinar:
Please join us for an educational webinar to review the clinical utility of our Epilepsy
Gene Panel Test. You must register in advance by clicking on the link beside the date you will attend.
March 5th, 2018: 3:00 pm EST (12:00 pm PST) • Register here
March 9th, 2018: 12:00 pm EST (9:00 am PST) • Register here
Webinar outline and presenters:
Dr. Bekim Sadikovic PhD, DABMGG, FACMG
Head of Molecular Genetics at London Health Sciences Centre (LHSC)
• Strategic direction and implementation of the LHSC epilepsy gene panel test at Dynacare
• Industry-leading approaches for clinical Next Generation Sequencing (NGS) and copy number
assessment bioinformatics
Dr. Hilary Racher PhD, DABMGG, FACMG
Scientific and Laboratory Director Impact Genetics, Dynacare
• Details of Epilepsy Gene Panel Test provided by Dynacare
Ms. Jaime Jessen MSc, CGC, CCGC
Medical Science Liaison Impact Genetics, Dynacare
• Guidelines for ordering hereditary Epilepsy Gene Panel Test
For test descriptions and ordering information please go to the Epilepsy section of our website.
For any additional information please contact us at 1-877-624-9769 or info@impactgenetics.com
February 9th, 2018
Impact Genetics Will Launch a Comprehensive Epilepsy Gene Panel Test on February 26th, 2018

Begining on February 26th, Impact Genetics will be offering London Health Sciences Centre’s comprehensive and
therapeutic Epilepsy panels, validated for clinical use, to all patients in Canada except Ontario. Next-generation
sequencing (NGS) technology will provide both sequence and copy number changes in a panel of 69 genes associated
with major epilepsy syndromes. Identifying the underlying genetic cause of epileptic seizures can inform treatment
options, predict prognosis and alert to secondary syndrome risks for patients and their family.
For test descriptions and ordering information please go to the Epilepsy section of our website.
For any additional information please contact us at 1-877-624-9769 or info@impactgenetics.com
November 28th, 2017
Impact Genetics Launches Tumor MMR Sequencing Test.
Impact Genetics is proud to announce that our Tumor MMR Sequencing Test is now available.
This test is used to both confirm and rule out Lynch Syndrome (biallelic somatic MMR cancer).
MMR analysis can be performed on a variety of tumor types (endometrial, colon, etc.).
Impact Genetics has over two decades of experience with paired tumor and germline genetic testing.
We are trusted by genetic counselors in oncology because of our experience with constitutional
variant analysis in tumors and expert interpretation.
Impact Genetics’ MMR somatic tumor testing includes:
– Advanced next-generation sequencing and copy number analysis (using MLPA) for MLH1,
MSH2, MSH6, PMS2 and EPCAM
– All reportable mutations detected in tumor are confirmed by an alternative method and
are investigated in the germline
– Tumor sample procurement and shipping
– Preauthorization services and flexible billing
MLH1/MSH2/MSH6/PMS2/EPCAM Somatic Tumor MMR Sequencing and Deletion/Duplication Test
is available to order on the LabCorp test menu (test number 481472).
For a detailed test description and ordering information go to the Tumor MMR Sequencing Test
section of our wesbite.
For additional information please contact us at 1-877-624-9769 or info@impactgenetics.com
March 23rd, 2017
Impact Genetics Launches BAP1 Tumor Predisposition Syndrome (BAP1-TPDS) Genetic Test.

Mutations in BAP1 cause a novel cancer syndrome which is characterized by early age onset of
benign melanocytic skin tumors, and later in life by a high incidence of mesothelioma, uveal melanoma,
cutaneous melanoma, renal cell carcinoma and additional cancers.
Now available:
– Full BAP1 gene sequencing, copy number assessment and expert reporting
and interpretation.
– Comprehensive pre-test and/or post-test genetic counseling, provided by LabCorp
Integrated Genetics, using telegenetics
– BAP1-TPDS can be ordered in combination with Impact Genetic’s Uveal Melanoma
Prognostic Genetic Test
Screening guidelines:
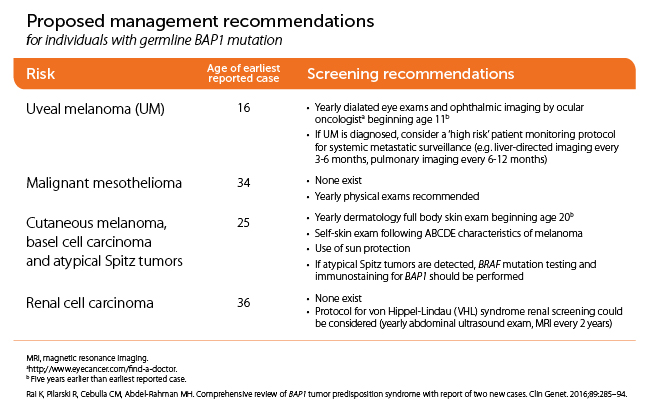
Rai K, Pilarkski R, Cibulla CM, Abdel-Rahman MH. Comprehensive review of BAP1 tumor predisposition syndrome with report of two new cases. Clin. Genet. 2016; 89: 285-94.
For additional information please contact us at 1-877-624-9769 or info@impactgenetics.com
March 20th, 2017
See you at ISOO 2017

Impact Genetics looks forward to seeing you at the 18th biennial International Society of Ocular Oncology
meeting in Sydney, Australia.
Dr. Hilary Racher and Ms. Jaime Jessen will be presenting 2 Short Case and 2 Free Paper Oral Presentations.
|
Jaime Jessen, BSc, MSc, CGC, CCGC
Dr. Hilary Racher, PhD, FCCMG, DABMGG |
Saturday, March 25, 2017 4:00 pm Jaime Jessen, BSc, MSc, CGC, CCGC Sunday, March 26, 2017 10:30 am Jaime Jessen, BSc, MSc, CGC, CCGC Sunday, March 26, 2017 5:10 pm Dr. Hilary Racher, PhD, FCCMG, DABMG Monday, March 27, 2017 3:30 pm Dr. Hilary Racher, PhD, FCCMG, DABMGG |
March 16th, 2017
Now Available: Faster test turn around times, updated and improved test order forms

Productivity and efficiency can be achieved only step by step with sustained hard work, relentless attention to details and insistence on the highest standards of quality and performance.
– J. R. D. Tata
At Impact Genetics we strive to continuously improve our service and test quality. We are excited to announce that our trusted gold standard molecular analysis and interpretation is faster than ever before.
UPDATED Test Turn Around Times
Retinoblastoma Genetic Test
Proband…………………………………………………………………………………………… 4 to 6 weeks
Known Familial Mutation……………………………………………………………………….. 2 to 3 weeks
Prenatal…………………………………………………………………………………………… 7 working days
Hereditary Hemorrhagic Telangiectasia (HHT) Genetic Test
Proband…………………………………………………………………………………………… 6 to 9 weeks
Known Familial Mutation……………………………………………………………………….. 2 to 3 weeks
Uveal Melanoma Prognostic Genetic Test
Impact Genetics UM Prognostic Test………………………………………………………….. 3 to 6 weeks
UPDATED Forms
Newly update and improved individual forms and test submission packages are available in the Order a Test section of the website.
For additional information please contact us at 1-877-624-9769 or at info@impactgenetics.com.
February 22nd, 2017
Research Brings Hope to People Living with a Rare Disease

Tuesday February 28th, 2017 6:30pm – 8:30pm
The Hospital for Sick Children
Peter Gilgan Research and Learning Tower Auditorium
686 Bay Street, Toronto
Ontario, Canada
Speaker: Meryl Acker BScH, MSc
Research impacts medical management in hereditary cancer predisposition
Speaker: Erica Spencer
Patient voice in hereditary cancer
Doors open at 6:30pm
Children Welcome
RSVP: torontofamilies@rarediseasefoundation.org
Reception to follow
*This event is independent from Impact Genetics.
February 13th, 2017
Uveal Melanoma Prognostic Genetic Test will now include SF3B1, EIF1AX, GNAQ and GNA11 sequencing for tumor confirmation in chromosomally normal tumor samples.

Impact Genetics has added two additional genes to our uveal melonoma tumor analysis SF3B1 and EIF1AX.
Recent publications (Yavuzyigitogly et al 2016 PMID: 26923342; Royer-Bertrand et al 2016 PMID: 27745836; Van Beek et al 2015 PMID: 26086698) have shown that disomy 3 patients with SF3B1 mutation are at increased risk to develop late-onset metastases, often presenting more than 5 years after diagnosis.
For disomy 3 samples that show normal MLPA and MSA results, mutation analysis will be performed on any remaining DNA.
SF3B1 will be preferentially reported allowing both tumor confirmation and enhanced prognostication for this unique patient group.
![]()
– Impact Genetics will offer a 5 gene uveal melanoma tumor panel; GNAQ, GNA11, SF3B1, EIF1AX and BAP1.
This will be orderable regardless of prognostic test result (tumor chromosomal status).
– Impact Genetics will offer BAP1 tumor predisposition syndrome germline (hereditary) analysis and genetic counseling services.
For additional information please contact us at 1-877-624-9769 or at info@impactgenetics.com.
February 13th, 2017
Important Shipping Notice for the Family Day Holiday, Monday February 20th, 2017.
Monday February 20th 2017 is Family Day in Ontario Canada, a statutory holiday. Our laboratory and offices will be closed, and not be receiving samples.
Regular hours for receiving samples resumes on Tuesday February 21st, 2017.
Please contact us directly with any questions you may have at 1-877-624-9769 or at info@impactgenetics.com.
December 22nd, 2016
Highlights of ocular research in 2016 from our Medical Advisors
2016 was a monumental year for ocular research. We would like to thank our medical advisors for their continued contribution to the advancement of ocular oncology. Here are some of the highlights:
“Genetic analysis of choroidal melanoma by MLPA or MSA following completion of PBR distinguishes between disomy 3 and monsomy 3 tumors and produces results that are predictive of metastasis–free survival”
– Damato et al. 2016. Prognostic Biopsy of Choroidal Melanoma After Proton Beam Radation Therapy. Opthalmology. 123(10) 2264-2265.
“Tumor sampling of small choroidal melanoma with a 27-guage vitreous cutter is safe and offers excellent biopsy yield for molecular prognostication when cancer prognosis is desired by patients”
– McCannel et al. 2016. Vitrectomy-assisted biopsy for molecular prognostication of choroidal melanoma 2mm or less in thickness with a 27-guage cutter. Retina. (published ahead of print).
“When a parent had retinoblastoma, prenatal molecular diagnosis with early-term delivery increased the likelihood of infants born with no detectable tumors, better vision outcomes, and less invasive therapy. Prenatal molecular diagnosis facilitates anticipatory planning for both the child and family.”
– Soliman et al. 2016. Prenatal versus Postnatal Screening for Familial Retinoblastoma. American Academy of Opthalmology. 123(12):2610-2617.
We look forward to seeing what 2017 brings in research!
November 20th, 2016
Genetic Counseling Awareness Week

Happy Genetic Counselling Awareness Week! Today marks the beginning of the 7th annual Genetic Counselling Awareness Week. This year’s theme is “Genetic Conditions: Not as Rare As You May Think”.
From November 20th to 26th, 2016, the Canadian Association of Genetic Counsellors (CAGC) is hosting Genetic Counselling Awareness Week and will be hosting events across Canada.
Genetic counsellors help people understand and adapt to complicated health-related information in a genetics setting. They can help individuals and families with rare genetic disorders in many ways, from providing support during the genetic testing process and explaining the complexities of genetic test results and diagnoses, to helping determine how newly discovered genes can be used in genetic testing. For genetic counsellors, rare may be unique, but it is not uncommon. This year, Genetic Counselling Awareness Week will focus on the idea that genetic conditions are more common than many may believe.
– Canadian Association of Genetic Counsellors
To learn more about Genetic Counselling Awareness Week, visit the CAGC’s Facebook page or follow them on Twitter @CAGC_ACCG.
November 18th, 2016
Important Shipping Notice for the 2016 / 2017 Holiday Season
Happy Holidays!
Our laboratory and offices will be closed for the Holidays and not be receiving samples on the following days:
Monday December 26th, 2016
Tuesday December 27th, 2016
Monday January 2nd, 2017
Regular hours for receiving samples will resume on Tuesday January 3rd, 2017.
Please contact us directly with any questions you may have at 1-877-624-9769 or at info@impactgenetics.com.
April 4th, 2016
Impact Genetics Tests Listed on LabCorp Test Menu.
April 4th, 2016 – Impact Genetics’ uveal melanoma and HHT tests are now available to be ordered through LabCorp. Customers in the United States can now send samples to Impact Genetics through LabCorp, improving access in larger institutions and in some cases, improving insurance coverage. LabCorp will handle specimen transport, insurance prior-authorizations and billing.
Contact us for details about the process.
May 28th, 2014
Impact Genetics Launches New Test for Uveal Melanoma.
May 28th, 2014 – Impact Genetics will launch a prognostic test for Uveal Melanoma on June 4th, 2014. This genetic test will identify risk of metastasis, indicating survival prognosis in patients with Uveal Melanoma.
“Prognostication may contribute to patient management, possibly encouraging a more aggressive approach if the tumor shows lethal genetic abnormalities”, says Dr. Bertil Damato, medical advisor to Impact Genetics and Director of the Ocular Oncology Service and Professor of Ophthalmology and Radiation Oncology, University of California, San Francisco.
“Prognostic tests identify patients at higher metastatic risk, who may benefit from the ongoing clinical trials for therapies of early metastases, and can also favourably impact the economics of metastatic surveillance”, says Dr. Hatem Krema, Director of the Ocular Oncology Service, University of Toronto, Canada.
For more information see our Uveal Melanoma Test Description:




